Chapter 9: Biological Safety
Effective Date: July 1, 1997 (Issue No. 2)
Last Updated: September, 2023
A. Introduction
The following safety rules are intended as guidelines. They were developed from various sources and specifically adopted from the guidelines issued by the National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC) and the National Cancer Institute (NCI) of the United States. Each lab supervisor should develop rules that are specific for his or her laboratory. All employees, visitors, contractors, etc., should be informed of these guidelines. Persons engaged in laboratories where there are biological hazards must also take into account chemical hazards or radiation hazards which are present and must recognise that these are special categories of risk. In all such cases it is necessary to ensure that any statutory safety regulations or codes of practice are observed and that adequate information is available relating to the hazards.
The purpose of these guidelines is to provide the information necessary for the safe conduct of teaching and research involving potentially hazardous biological materials. These guidelines have been assembled by HSEO to ensure that researchers who work with biohazardous materials have access to and understand the information needed to perform their work safely.
The essence of these guidelines is that the greater the risk of exposure to a biological agent, the greater the caution needed to prevent exposure. Work with biological agents has been classified according to four “Biosafety Levels”. These levels correspond to the perceived risk of exposure to biohazardous agents: the higher the biosafety level number, the higher the risk. As is true in the case of carcinogens, evaluation of risk takes into consideration both the intrinsic hazard of the agent and the concentration of it in the samples handled. The application of these principles of risk assessment to categorization of biohazards is presented in the summary tables provided in Section D, entitled “Description of Biosafety Levels”. The primary requirements for working at Biosafety Levels 1 and 2 are given in Section E1 and E2. Information on decontamination, containment, and medical surveillance is presented in later sections. Contact HSEO for more detailed discussion of specific etiologic agents and laboratory practices.
B. Planning
The user must evaluate each task in which biological agents are to be used in order to determine the associated risks. This evaluation must include a consideration of the virulence of the organisms. Additionally, eventual disposal options and waste minimisation techniques should be evaluated in the planning stage. Further, the tasks and organisms involved should be reviewed by a knowledgeable person in advance of the operation.
To conform with the most widely accepted classification for biosafety levels, these guidelines use classification methods adopted from documents issued by the National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC) and the National Cancer Institute (NCI). These classification methods assign biohazardous and carcinogenic agents to Biological Safety Levels 1, 2, 3 and 4, (BSL1-4) which are generally equivalent to biological safety levels BL1, BL2, BL3 and BL4, required by the NIH for recombinant DNA work.
The following discussion describes each of the four levels of containment in an abbreviated form to provide a general idea of the work that should be conducted under these categories. The fourth level involves agents which cannot be handled at the levels of containment usually available at academic institutions. HKUST currently also does not have suitable facility for Biosfaety Level 3 work, even though the requirements can be attained by upgrading existing facilities when necessary. The salient features of laboratory practices, special equipment, and facilities appropriate for each of these levels of biosafety are summarized in Table 1. The relationships between levels of containment assigned to work with recombinant DNA, etiologic agents, oncogenic viruses, and carcinogens are summarized in Table 2.
It is the duty of each worker or student to co-operate in the prevention of accidents. It must be strongly emphasized that those personnel who fail to take adequate precautions may endanger not only themselves but others as well.
C. Responsibilities
Principal investigators/supervisors are responsible for
- determining the appropriate biosafety level
- establishing safe procedures
- providing proper protective equipment needed in safe handling of biological agents
- instructing their personnel as to the possible hazards, the safety precautions, waste handling procedures, the consequences of an accident, and the actions to take in case of an accident
- assuring that employees and students are held accountable for the agents and equipment that they work with.
Employees and students are required to learn and understand the hazards of the organisms as well as the chemicals they work with. They should also be familiar with the operational features of lab equipment and follow all precautions applicable to each task. In case of unexpected malfunctioning, damage, or injury, the employee and student should act to protect himself and others in the area. He or she should also report to the supervisor any unsafe or hazardous condition in the area. In case of a job/program transfer or termination, employees or students must properly dispose of or transfer all chemicals and assigned equipment to another responsible party before leaving.
The Health, Safety and Environment Office (HSEO) assists supervisors, employees and students in maintaining safe work areas by providing information on the hazardous properties of materials, recommending methods for controlling them, and by monitoring the work environment. In addition, the HSEO offers formal education and training courses on the recognition, evaluation and control of various safety hazards.
D. Description of Biosafety Levels
Biological safety levels are categorized according to 4 levels and are indicated as BSL-1, BSL-2, BSL-3 and BSL-4. BSL-2 level work is the highest level of biohazardous work allowed at HKUST (i.e. no BSL-4 or BSL-3 work is allowed). Biosafety levels are determined by several considerations. The initial factor to be considered is the classification of the biohazardous agent being handled. After this determination has been made, additional considerations are:
- whether or not the agent has been or is being genetically modified and lacks an established track record relative to safe handling in laboratories.
- whether or not animals are being used in the experiment.
- how the agent is being handled within the laboratory.
TABLE 1 Summary of Recommended Biosafety Levels for Infectious Agents
Reproduced from the CDC-NIH publication “Biosafety in Microbiological and Biomedical laboratories.”
| Biosafety Level | Practices/Technique | Safety Equipment | Facility |
| 1 | Standard microbiological | None: Primary containment provided by adherence to standard laboratory practices during open bench operations. | Basic |
| 2 | Level 1 practices plus: laboratory coats; protective gloves; biohazard warning signs as indicated; limited access; decontamination of all infectious wastes | Partial containment equipment (i.e. Class I or II Biological Safety Cabinets) used to conduct mechanical and manipulative procedures with high aerosol potential that may increase the risk of exposure to personnel. | Basic |
| 3 | Level 2 practices plus: special laboratory clothing; controlled access | Partial containment equipment used for all manipulations of infectious materials. | Containment |
| 4 | Level 3 practices plus: entrance through change room where street clothing is removed and laboratory clothing is put on; shower on exit; all wastes are decontaminated on exit from the facility | Maximum containment equipment (i.e. Class III biological safety cabinet containment or partial containment equipment in combination with full-body, air-supplied, positive-pressure personal suit) used for all procedures and activities. | Maximum Containment
|
TABLE 2 Regulatory Classification of biohazards and carcinogens and corresponding biosafety levels
| Agent | Classification | Biological Safety Level |
| Etiologic Agents | Class 1 Class 2 Class 3 Class 4 |
1 2 3 (not authorized at HKUST) 4 (not authorized at HKUST) |
| Recombinant DNA | Risk Group 1 Risk Group 2 Risk Group 3 Risk Group 4 |
1 2 3 (not authorized at HKUST) 4 (not authorized at HKUST) |
| Oncogenic Viruses | Low Risk Moderate Riska High Risk b |
1 2,3 |
| Regulated Carcinogens | Category 3 Category 2 Category 1 |
2 2 3 |
a Investigators working with moderate risk oncogenic viruses may be advised to comply with certain Safety Level 3 guidelines as determined by HSEO.
b At the present time there are no known oncogenic viruses classified as high risk. Containment would be recommended on a case-by-case basis by HSEO.
E. Recommended Procedures for Work
1. Biosafety Level 1
Most laboratories work with non-pathogens. The recombinant DNA work is generally with systems which are well within the NIH guidelines for Biosafety Level 1, requiring only simple procedures necessary for sterile work and standard microbiological laboratory practices. Similarly, most work with mammalian cell cultures is classified as Biosafety Level 1. A summary of the most salient features of “good” microbiological laboratory practices is given below. More detailed instructions and suggestions on the handling of biohazards, including disinfectants, supplies and equipment for the handling of biological materials, are found in later sections of this guide.
1.1 Use Standard Sterile Work Practice
Standard laboratory practice for the handling of nonpathogenic microorganisms includes all the measures required for sterile work, for example:
- Surfaces where work is to be conducted should be decontaminated with a disinfectant (e.g. Mikro-Quat, Amphyl, or bleach) before and after any manipulations.
- A Bunsen burner should be available for flaming all glass containers and pipettes during the transfer of liquids and materials.
1.2 Autoclave Contaminated Liquid and Solid Waste
All materials and supplies used during an experiment which have come in contact with microorganisms or mammalian cells except materials and supplies containing radioactive materials or carcinogens should be autoclaved. This includes flasks used to grow cells, pipettes used to transfer cell suspensions, pellets obtained during centrifugation after disruption of cells, and cell extracts prepared to carry out enzymatic assays. Also it is proper to autoclave all Petri plates used to grow bacteria or culture vessels from mammalian cell culture, materials used to wipe or clean surfaces, and other supplies that have contacted microorganisms or mammalian cells. Disposable tubes, pipettes, and other materials should be used whenever possible.
Materials to be autoclaved should be placed in leak proof containers and properly labeled with “autoclave” tape. Glassware should be autoclaved separately from disposable waste, rinsed following autoclaving, and then sent through the normal wash cycle. Containers should be available in each lab for the disposal of liquids and solids which are contaminated with microorganisms or mammalian cells. Autoclavable bags supported on holders are good for discarding most solid waste and small volumes of liquid waste. For larger volumes of liquid waste closed flasks or autoclavable bottles can be used. Disposable waste that has been autoclaved may be combined with normal waste.
Materials for which autoclaving is not practical should be decontaminated using one of the disinfectants discussed in Section F3.
1.3 Discarding Carcinogenic or Radioactive Waste
Waste from experiments in which radioactive or carcinogenic compounds are used in conjunction with biological materials should be discarded with the radioactive or chemical waste. Where possible these materials should be chemically decontaminated with respect to the biological agent(s) present (see Section F). NEVER decontaminate biohazardous waste containing carcinogens or radioactive materials by autoclaving; contamination of personnel and equipment may result.
1.4 Do Not Eat, Drink, Smoke or Store Food in Laboratory
Eating, drinking, smoking or storage of food should not be allowed in the laboratory.
1.5 Use Pipetting Devices
Mechanical pipetting devices must be used; mouth pipetting is prohibited.
1.6 Close Laboratory Doors
Laboratory doors should be kept closed when experiments are in progress.
1.7 Wash Hands
All persons should wash their hands after handling biological materials.
1.8 Label Vessels
All vessels containing viable biological materials should be properly labeled to provide information to others in case of breakage and spillage. Label information should include: organism present, approximate concentration, special features if any, and responsible investigator.
2. Biosafety Level 2
Agents present in work classified at Biosafety Level 2 present only moderate risks to the worker because they are not highly infectious in normal adults and are not present at high concentrations or in large volumes. The procedures to be used when working with these materials are simple ones intended to reduce the possibility of accidental biohazard exposure of researchers and those working around them.
2.1 Follow Biohazard Level 1 Procedures
Follow all procedures recommended for Biosafety Level 1.
2.2 Put Biohazard Warning Signs on Doors
When infectious materials are present in a laboratory, a biohazard warning sign (download here) should be placed on all laboratory access doors. The sign should indicate the biohazard present, the person(s) responsible for the work, and any restrictions on access to the room.
2.3 Put Biohazard Symbol on Samples and Equipment and Waste Containers
Labels with the biohazard symbol (download here) should be placed on all samples and on equipment used with these samples, such as incubators, refrigerators, centrifuges, and waste containers.
2.4 Limit Laboratory Access
Access to the laboratory during experiments should be limited to persons informed of the nature of the work conducted. Children under 18 years of age and immune-suppressed persons should be denied access to the room when experiments are in progress.
2.5 Wear Lab Coats
Lab coats must be worn while working in the laboratory with these materials or while others are working with them. Lab coats are not to be worn in non-lab areas.
2.6 Increase Hand Washing
Hands should be washed after handling potentially infectious materials and before leaving the laboratory.
2.7 Wear Gloves
Gloves must be worn to avoid skin contamination with potentially infectious materials contained in viable samples of human or primate blood, serum, semen, or other tissues or bodily fluids. Gloves are to be removed and disposed of before using common-use items, such as telephones and computers, or moving to non-laboratory areas of the building.
2.8 Always Use Pipetting Devices
Mechanical pipetting devices must be used. Mouth pipetting is prohibited.
2.9 Limit Use of Syringes and Hypodermic Needles
Use of syringes and hypodermic needles should be kept to the absolute minimum, i.e. only for injection and aspiration of fluids from diaphragm bottles or animals. Only needle-locking syringes or disposable syringe-needle units should be used for these purposes. To avoid accidental puncture wounds, needles should not be re-sheathed, bended, or clipped after use. Intact syringe and needle assemblies should be placed in a puncture-resistant sharps container. As a general practice, all sharps containers are decontaminated and disposed of by incineration with carcinogenic/radioactive solid wastes.
2.10 Avoid Generating Aerosols
Procedures that may generate aerosols should be avoided when possible. Those that require open vessels, such as pipetting, opening ampoules of lyophilized material, sonicating and using a blender, must be conducted in a biological safety cabinet (class I or II). Centrifugation, vortexing, or other procedures performed in properly closed non-breakable vessels may be conducted on the open lab bench.
Poorly sealed tubes or Eppendorf tubes can generate aerosols when spun in table top centrifuges/microfuges. These operations should be performed in a biological safety cabinet. Alternatively, sealed rotors can be used in centrifuges, and the rotor opened in a biological safety cabinet.
Wearing of masks is not recommended. They are unnecessary when a biological safety cabinet is used. Masks cannot substitute for a biological safety cabinet because they provide limited protection for personnel and no protection of the environment.
2.11 Conduct Certain Types of Work in Biological Safety Cabinet
Work that may generate aerosols (see E2.10 above) must be performed in a biological safety cabinet. When high concentrations or large volumes of certain infectious agents, such as the hepatitis virus, are used, all work should be conducted in a biological safety cabinet (Class I or II). Such materials may be centrifuged in the open laboratory if sealed heads or centrifuge safety cups are used and are opened only in the biological safety cabinet. Consult the CDC-NIH publication Biosafety in Microbiological and Biomedical Laboratories, 4th Ed (http://www.cdc.gov/od/ohs/biosfty/bmbl4/bmbl4toc.htm) or HSEO for guidance on the agents included in this restriction.
2.12 Autoclave Contaminated Liquid and Solid Waste Unless Co-contaminated with Radioactive or Carcinogenic Materials
All contaminated liquid and solid waste except waste containing radioactive materials or carcinogens must be decontaminated by autoclaving before disposal. Disposable tubes, pipets, culture vessels, and other disposable materials should be used whenever possible. Glassware to be reused should be autoclaved separately from disposable waste, rinsed and sent through the normal wash cycle. Contaminated material that is to be decontaminated by autoclaving at a site away from the laboratory should be placed in a durable leak-proof container, such as an autoclavable bag, which is closed and marked with “autoclave” tape with a biohazard label, before being removed from the laboratory. See Section F4.1.b.
When carcinogens and/or radioactive materials are also present, waste should be chemically disinfected prior to handling as a chemical and/or radioactive waste. Identify biohazardous materials in the description of the waste. Care should be taken to employ a decontamination procedure that does not interact with the sample in a way that produces toxic products, e.g. do not add bleach to samples that contain radioactive iodine, or formaldehyde to samples containing HCl. NEVER decontaminate biohazardous waste containing carcinogens or radioactive materials by autoclaving. Contamination of both personnel and equipment may result. For further information on biohazardous samples that also contain carcinogens or radioisotopes, see Sections E5 and E6, respectively.
In the course of an experimental procedure, infectious materials are often inactivated, and subsequent work is derated to Biosafety Level 1. See Section G for examples of procedures/ treatments that inactivate many biohazardous agents. For additional information on this aspect of working with biohazards, consult your supervisor and/or members of HSEO.
2.13 Keep Animals Out of the Laboratory
Animals not involved in the work being performed should not be in the laboratory.
2.14 Collect and Store Baseline Serum Samples
Baseline serum samples should be collected and stored frozen for all laboratory personnel and others at risk of exposure to viable agents in samples of human or other primate tissues and bodily fluids, such as blood, serum, and semen, and all personnel that handle experimental animals. See Section I.
3. Biosafety Level 3
Currently, no work classified at Biosafety Level 3 is conducted at HKUST. Work with agents that cause serious or potentially lethal disease following inhalation exposure is classified at this level. Autoinoculation and ingestion of these agents are also considered hazardous. Also classified at Level 3 is work that involves high concentrations, large volumes, or aerosol production of certain agents that are otherwise classified at Biosafety Level 2 and recombinant DNA work involving Class 3 agents, such as Mycobacterium tuberculosis, Histoplasmosis capsulatum, Arbo viruses or rabies virus. Access to Biosafety Level 3 facilities is controlled, and personnel wear special protective clothing. Facilities used for work at this level are designed to facilitate containment. Extensive modification of most current facilities at HKUST would be needed to achieve the required level of containment.
4. Biosafety Level 4
No work classified at Biosafety Level 4 is conducted at HKUST. Such work would involve dangerous and exotic agents which present a high risk of life-threatening disease. Examples of such agents are Lassa fever virus, Kyasanur Forest disease virus, and Marburg virus, and recombinant DNA studies involving genes for toxins that are highly toxic for vertebrates. Stringent laboratory practices are required as well as maximum containment equipment and facilities. No such facilities are currently available at HKUST, or in Hong Kong, and work at this level is not contemplated.
5. Biohazardous Samples That Also Contain Carcinogenic Materials
5.1 Experimental Requirements
Chemical carcinogens are divided into three categories, 1, 2 and 3, listed in order of decreasing carcinogenic potency. Category 1 chemical carcinogens are handled in a glove box until they are diluted to specified concentrations. Once diluted, category 1 carcinogens can be handled in the same manner as category 2 and 3 carcinogens, i.e. within a ventilated enclosure which does not recirculate the major part of the exhaust volume. A chemical fume hood or a type 2B biological safety cabinet is suitable. When working with a biological sample, the type 2B biological safety cabinet is the containment equipment of choice since it affords protection against both biological and chemical agents, as well as preventing contamination of the experiment.
5.2 Disposal Requirements
Materials that are co-contaminated with carcinogens and biohazardous materials should be disposed in one of two ways depending on whether or not it is possible to inactivate the carcinogen(s):
- If it is possible to inactivate the carcinogen(s) present in the waste, do so. Then decontaminate the waste with respect to the biohazard present by autoclaving. Contact HSEO for further information.
- If it is not possible to inactivate the carcinogen(s), chemically decontaminate the materials with respect to the biohazard present if possible. Do not use chlorine as the disinfectant. Dispose of the waste in an appropriately labeled chemical waste container. Co-contaminated samples should be double bagged. This waste will then be handled by HSEO as appropriate for the contents. Include biohazard materials in the list of contents.
CAUTION NEVER decontaminate biohazardous waste containing active carcinogens by autoclaving. Contamination of both personnel and equipment may result.
5.3 Substitute for Carcinogenic Agents
It is always advisable to seek suitable substitute for carcinogenic agents used in experimental procedures. One agent commonly used in molecular biology work is ethidium bromide (EtBr, also known as homidium bromide), which is highly toxic if inhaled, and is a known mutagen. Alternative DNA dyes that are much safer to use are now commercially available, such as SYBR Safe and GelRed. Even though the cost of these alternative dyes is slightly higher, the benefit of significantly reduced chemical hazard clearly outweighs the additional cost. Users of EtBr should seriously consider switching to an alternative DNA dye. Further information about the alternative dyes can be obtained from these 2 Safetywise articles: article 1/ article 2.
6. Biohazardous Samples That Also Contain Radioisotopes
6.1 Experimental Requirements
Chapter 10 of the HSEO’s Safety and Environmental Protection Manual specifies requirements for work with radioactive substances. For low hazard operations, requirements can generally be satisfied if the experiments are carried out under either Biosafety Levels 1 or 2. For moderate hazard operations, requirements include the use of a non-recirculating ventilated enclosure. Since most biological safety cabinets recirculate a portion of the exhaust air, they do not satisfy this requirement. A special non-recirculating biological safety cabinet connected to the exhaust should be used. This hazard level also requires the use of a totally closed work area only accessible to project personnel.
Examples of the activity of radioisotopes commonly employed in biological research are given in the table below. For additional information contact HSEO.
6.2 Radioisotopes Commonly Used
TABLE 3 Maximum radionuclide activity authorized in a biological safety cabinet compared to activity common in biological research
| Radionuclide | Maximum activity authorized for work with radionuclide solutions in a biological safety cabinet a | Activity commonly used in biological research b |
| 3 H | 1,000 mCi | 10 mCi |
| 14 C | 500 mCi | 10 mCi |
| 3 2 P | 100 mCi | 2 mCi |
| 35 S | 400 mCi | 1 mCi |
| 125 I | 10 mCi | – |
| 131 I | 10 mCi | – |
a This activity level generally corresponds with Biosafety level 2. The activities listed are for radionuclide solutions with activity concentration less than 100 mCi/mL. For solutions exceeding 100 mCi/mL, contact HSEO for a work area evaluation.
bThis activity level generally corresponds with Biosafety Level 1. Good laboratory safety practices are essential.
The values in Table 3 are guidelines. Prior to any work involving radioactive material, HSEO should be contacted for an evaluation of the proposed handling procedures and work area.
6.3 Disposal Requirements
Materials that are co-contaminated with radioisotopes and biohazardous materials should first be chemically decontaminated with respect to the biohazard present, if possible. Do not use chlorine as the disinfectant. The waste should be placed in an appropriately labeled radioactive waste container. Co-contaminated samples should be double bagged. This waste will be handled by HSEO as appropriate for the contents. Identify biohazardous materials in the list of contents.
CAUTION: NEVER decontaminate biohazardous waste containing carcinogens by autoclaving. Contamination of both personnel and equipment may result.
F. Decontamination
1. GENERAL PROCEDURES FOR DECONTAMINATION OF WASTE
The most consistent and effective way to decontaminate biological waste is autoclaving. Autoclaving is fast and removes any ambiguity as to whether the decontamination process has been completed. For these reasons, and to eliminate concerns for health and safety among our staff and the community, we recommend that all biological materials except materials containing radioactive materials, carcinogens or hazardous volatile chemicals be disinfected by autoclaving prior to disposal, even though Biosafety Level 1 materials do not strictly require this method of disinfection.
All laboratories generating potentially biohazardous waste need to maintain appropriate containers to dispose, carry, and autoclave this waste. These items are available from CLS. It is expected that most of the waste generated will be disposable. For glassware and reusable items, it will be the responsibility of each laboratory to maintain separate autoclavable containers in which these materials can be autoclaved. After being autoclaved, these reusables should be rinsed before they are sent through the normal washing cycle.
For decontamination of table tops and surfaces, it is recommended that liquid decontaminating agents such as amphyl, broccal or other liquid disinfectants listed in Section F3 be used. As with the autoclave bags and containers, these items are available through the CLS stock room.
Waste which contains radioactive and/or carcinogenic or other hazardous compounds in conjunction with any biological samples should, if possible, be disinfected by chemical means (see Section F3), then discarded in appropriately labeled radioactive/chemical waste containers, i.e. biohazardous materials listed in addition to hazardous chemical and/or radioisotopes. This waste will be handled appropriately by HSEO. NEVER decontaminate biohazardous waste containing carcinogens, hazardous volatile chemicals, or radioisotopes by autoclaving. Contamination of both personnel and equipment may result. For further information on biohazardous samples that also contain carcinogens, hazardous chemicals or radioisotopes, see Sections E5 and E6, respectively.
2. HANDLING SPILLS
In any laboratory operation, the possibility of a spill exists. A thorough understanding of the potential hazards of the experiment as well as careful planning of a spill response protocol will help minimize personal injury and property damage.
While the severity of the accident will dictate the response required, the following general approach is recommended.
2.1 FIRST PRIORITY: PROTECT PERSONNEL
-
Notify your supervisor and the Security Control Centre (8999) from the nearest phone. Do not contaminate the phone.
-
Notify all personnel in the immediate area. Evacuate the immediate room or area if the accident is hazardous to others or if you are in doubt about the extent of the hazard.
- If the agent involved may be hazardous when inhaled, hold your breath as much as possible. Remove contaminated clothing and shoes and leave the area. Wash hands, face and other contaminated portions of the body with appropriate disinfectant and soap. If eyes have been contaminated, flush with water for 15 minutes.
- Secure the area of the spill and prevent people from entering the area. Place a sign at the entrance specifying the type of accident, the agent(s) involved, date and time of the accident, name of person to contact prior to entering the area.
2.2 SECOND PRIORITY: PROTECT EQUIPMENT AND FACILITY THROUGH PROPER CLEANUP AND DECONTAMINATION
-
Proceed only after consultation with, and approval from HSEO.
-
Re-enter the area after allowing at least 30 minutes for aerosols to settle.
- If the spill takes place in the open laboratory, decontamination may range from flooding the area with an appropriate disinfectant to fumigating the entire room with disinfectant chemical vapor, depending on the nature and scale of the spill. Use of appropriate personal protective equipment varies according to the level of hazard involved. In general, it may involve some or all of the following: coveralls, gloves, shoe covers, head cover, and respiratory protection.
- If the spill takes place within a biological safety cabinet, initiate chemical disinfection procedures while operating the cabinet ventilation system to prevent the escape of the contaminants from the cabinet. Appropriate gloves should be worn. Use sufficient disinfectant to assure the disinfection of the drain pans and catch basins below the work surface. Lift the front exhaust grill and tray and wipe all surfaces. Wipe the catch basin and drain the disinfectant into a container. Items used during the cleanup should be autoclaved prior to disposal or normal cleaning. This procedure will not disinfect the filters, blower, air ducts or other interior parts of the cabinet. Contact HSEO for decontamination of the entire cabinet.
- If the spill involves a radioactive biohazardous material, the cleanup procedure may have to be modified depending on the relative risk assessment of the biological and radiological hazards involved. Contact HSEO for specific details.
- If the spill involves a carcinogenic biohazardous material, special equipment and/or reagents may be required. Contact HSEO for specific details.
3. CHEMICAL DISINFECTANTS
Inactivation of microorganisms by chemical disinfectants may involve one or more of the following mechanisms: coagulation and denaturation of protein, lysis, and inactivation or destruction of an essential enzyme. Chemical disinfectants can be found in either gaseous or liquid form. Vapor and gaseous disinfectants are primarily useful in closed systems for sterilization purposes. The effectiveness of liquid chemical disinfectants hinges upon factors such as concentration, pH, temperature, contact time, penetration and dispersion. For these reasons, even when used under highly favorable conditions, complete reliance should not be placed on liquid disinfectants when sterility is required. Significant properties of common chemical disinfectants are summarized below. Be alert to the flammable nature of some disinfectants, and use accordingly.
3.1 ALCOHOL
Ethyl or isopropyl alcohol in a concentration of 70-80% by weight is often used as a disinfectant. Alcohols denature proteins and are somewhat slow in their germicidal action. They are good general-use disinfectants, but they exhibit no activity against bacterial spores.
3.2 CHLORINE
This halogen is a universal decontaminant active against all microorganisms, including bacterial spores. Chlorine combines with protein and rapidly decreases in concentration in its presence. Free, available chlorine is an active element. It is a strong oxidizing agent, corrosive to metals. Chlorine solutions will gradually lose strength, so fresh solutions must be prepared frequently. Sodium hypochlorite is usually used as a base for chlorine decontaminants. An excellent decontaminant can be prepared from household or laundry bleach. These bleaches usually contain 5.25% available chlorine or 52,500 ppm. Diluted 1 to 100, the resulting solution contains 525 ppm of available chlorine, and if a non-ionic detergent such as Naccanol is added in a concentration of about 0.7%, a very good decontaminant is created.
3.3 IODINE
The disinfectant characteristics of chlorine and iodine are similar. One of the most popular groups of disinfectants used in the laboratory is the iodophors, and of this Wescodyne is perhaps the most widely used. The dilution of Wescodyne recommended by the manufacturer ranges from 1 oz in 5 gal of water yielding 25 ppm of available iodine to 3 oz in 5 gal yielding 75 ppm. At 75 ppm, the concentration of free iodine is 0.0075%. This small amount can be rapidly taken up by extraneous protein. Clean surfaces or clear water can be effectively treated by 75 ppm available iodine, but difficulties may be experienced if any appreciable amount of protein is present. For washing hands or for use as a sporicide, it is recommended that Wescodyne be diluted 1 to 10, or 10% in 50% ethyl alcohol. This will yield 1,600 ppm of available iodine; a relatively rapid inactivation of all microorganisms will occur.
3.4 QUATERNARY AMMONIUM COMPOUNDS OR QUATS
After years of testing and use, there is still considerable controversy about the efficiency of the “Quats” as disinfectants. These cationic detergents are strongly surface-active, and this detergent property makes them good surface cleaners. They will attach to protein; so, dilute solutions of Quats will lose effectiveness in the presence of proteins. The Quats tend to clump microorganisms and are neutralized by anionic detergents, such as soap. They are bacteriostatic, tuberculostatic, sporostatic, fungistatic, and algistatic at low concentrations. They are bactericidal, fungicidal, algicidal, and virucidal against lipophilic viruses at medium concentrations, but they are not tuberculocidal, sporocidal, or virucidal against hydrophilic viruses even at high concentrations. The Quats have the advantages of being odorless, nonstaining, noncorrosive to metals, stable, inexpensive and relatively non-toxic. Caution should be used when handling concentrated Quats; even a small droplet splashed into the eyes may cause blindness. Be sure to wear safety glasses and proper personal protective equipment when handling these disinfectants.
3.5 FORMALDEHYDE
Formaldehyde for use as a disinfectant is usually marketed at about 37% concentration of the gas in water solution, referred to as formalin, or as a solid polymerized compound, paraformaldehyde. Formaldehyde in a concentration of 5% active ingredient is an effective liquid disinfectant. Formaldehyde loses considerable disinfectant activity at refrigeration temperature, 4oC. It is pungent and irritating in odor and is a confirmed human carcinogen. Formaldehyde vapor generated from formaldehyde solution is an effective space disinfectant for sterilizing rooms or buildings. Formaldehyde gas can be generated by heating paraformaldehyde to de-polymerise it. In the absence of high moisture content in air, the gaseous formaldehyde released forms less polymerized residues on surfaces and requires less time to clear treated areas of fumes than formaldehyde released in the vapor state. Formaldehyde vapors and gas can elicit hypersensitivity and irritation. They are also toxic and lachrymatory. Dilutions should be made in a chemical fume hood. Respiratory protection may be necessary. Formaldehyde vapours are flammable and proper precautions should be exercised to prevent explosion when working with this material.
3.6 PHENOL
Phenol itself is not often used as a disinfectant. The odor is somewhat unpleasant, and a gummy residue remains on treated surfaces. This is especially true during steam sterilization. Although phenol itself may not be in widespread use, phenol homologs and phenolic compounds are bases of a number of popular disinfectants, such as Lysol. The phenolic compounds are effective disinfectants against some viruses, rickettsiae, fungi and vegetative bacteria. The phenolics are not effective in ordinary use against bacterial spores. Concentrated phenolics should be used carefully; even a small droplet splashed into the eyes may cause blindness. Phenolics are readily absorbed by the skin; splashes can lead to both local irritation, severe burns, and systemic poisoning. Consequently, safety glasses and other proper personal protective equipment should be worn.
3.7 OTHER VAPORS AND GASES
Besides formaldehyde, other decontaminant vapors and gases include ethylene oxide, peroxyacetic acid, hydrogen peroxide, beta-propiolactone, methyl bromide, and ethylene amine. When they are used in closed systems and under controlled conditions of temperature and humidity, excellent decontamination can be obtained. Ethylene oxide is convenient to use, versatile and noncorrosive, however, it is highly toxic and has been identified as a suspected carcinogen and its residuals must be removed by aeration. Peroxyacetic acid is corrosive for metal and rubber. Beta-propiolactone in the vapor form acts rapidly against bacteria, rickettsia and viruses. It has a half-life of 3-5 hours when mixed with water, is easily neutralized with water and lends itself to removal by aeration. However, beta-propiolactone is also a suspected carcinogen. Vaporized hydrogen peroxide (VPH) has been used in pharmaceutical industries for disinfection, and has the advantage of not leaving any undesirable residue behind, because it decomposes to oxygen and water. It has also been used for disinfection of biological safety cabinets. VPH has a potential of being applied to other disinfection applications.
3.8 CAUTIONS REQUIRED WHEN APPLYING CHEMICAL DISINFECTANTS
When handling concentrated stock solutions of certain disinfectants, be aware of the potential hazards and exercise due caution. Concentrated quaternary and phenolic disinfectants are particularly harmful to the eyes. Even a small droplet splashed in the eyes may cause blindness. Absorption of phenolic compounds by the skin can lead to local irritation, severe burns, and to systemic poisoning. Chronic or prolonged exposure to phenol may cause headache, dizziness, difficulty in swallowing, diarrhea, vomiting, shock, convulsions, and death. Safety glasses and proper personal protective clothing should be worn to avoid corrosion and depigmentation of the skin. Good ventilation is required when working with phenol to minimize inhalation.
Vapor of formaldehyde can elicit hypersensitivity and irritation. It is also toxic. When making dilutions, work in a chemical fume hood. Respiratory protection may be necessary. Formaldehyde vapor is flammable and explosions can result so proper precaution should be exercised. In addition to considering the target organism, when choosing a chemical disinfectant also consider the potential chemical reactions between the disinfectant and the chemical components of the material to be disinfected. For example, the use of a chlorine-based disinfectant in a system containing radioactive iodine will result in the release of gaseous radioactive iodine. As a second example, mixing formaldehyde with a system containing hydrochloric acid will result in the generation of bis(chloro-methyl)ether, a potent human carcinogen.
Any question on the selection of an appropriate disinfectant should be directed to HSEO.
Pertinent characteristics and potential applications for several categories of chemical disinfectants most likely to be used in the biological laboratory are summarized in Table 4. The suggested practical concentrations and contact times may differ markedly from the manufacturer’s recommendations. A high degree of protection of microorganisms by organic matrices has been assumed, and a sterile state has not been assumed to result from application of the indicated concentrations and contact times. It should be emphasized that these data only indicate efficacy under artificial test conditions. The efficacy of any of the disinfectants should be conclusively determined by individual investigators. It is readily evident from Table 4 that each of the disinfectants has a range of advantages and disadvantages as well as a range of potential for inactivation of a diverse microflora. Equally evident is the need for compromise as an alternative to maintaining a large collection of different disinfectants.
TABLE 4 SUMMARY OF PRACTICAL APPLICATIONS OF CHEMICAL DISINFECTANTS

4. PHYSICAL AGENTS OF DISINFECTION
4.1.A. HEAT
The application of heat, either moist or dry, is recommended as the most effective method of sterilization. Steam at 121oC under pressure in the autoclave is the most convenient method of rapidly achieving sterility. Dry heat at 160o to 170oC for periods of 2 to 4 hours is suitable for destruction of viable agents on impermeable nonorganic materials such as glass, but is not reliable in even thin layers of organic or inorganic material that can act as insulation. Incineration kills microorganisms and serves as an efficient means for disposal. Sample requirements for steam autoclaving of some materials are listed below:
-Laundry: 121oC for 30 minutes with 15 minutes pre-vacuum of 27 inches of Hg.
-Trash: 121oC for 1 hour with 15 minutes pre-vacuum of 27 inches of Hg.
-Glassware: 121oC for 1 hour with 15 minutes pre-vacuum of 27 inches Hg.
-Liquids: 121oC for 1 hour for each gallon.
4.1 B. AUTOCLAVE USE
Safe and effective use of autoclaves for disinfection requires:
-
Use of proper bags and containers, with adequate openings for steam penetration and pressure release.
- Water should be added to bags containing dry materials such as paper goods.
- Caps on flasks and tubes should be left off or loosely fastened.
- Biohazard level 2 materials should be double bagged.
- Label materials with experimenter’s name, lab room number, autoclave status, and, if appropriate, presence of biohazard level 2 materials.
- Exclude radioactive or carcinogenic materials, or materials containing more than trace amounts of solvents or corrosive materials, such as phenol, chloroform, trichloroacetic acid, chlorox.
- Load the autoclave properly. Do not overload or pack too tightly.
- Carefully remove materials at the end of the autoclave cycle. All pressure should have been released. On opening the autoclave, hands, arms, and face should be protected from released steam; gloves, lab coat, and protective eye wear should be worn.
- Always check temperature and pressure readings and/or records of autoclave to ensure the target levels are reached. Keep records of autoclave runs as proof of proper waste disinfection.Conduct periodic performance test using thermophilic bacterial spores test kits available from CLS. Keep records as proof of proper waste management practice.
4.2 NON-IONIZING RADIATION
Ultraviolet radiation (UV) is a practical tool for inactivating viruses, mycoplasmas, bacteria, and fungi. UV is especially useful for the destruction of airborne microorganisms on exposed surfaces or for the treatment of products of unstable composition that cannot be treated by conventional methods. Its usefulness as a sanitizer is limited by its low penetrating power. UV is primarily used in air-locks, animal holding areas, ventilated cabinets, and laboratory rooms during periods of non-occupancy to reduce the levels of viable airborne microorganisms and to maintain good air hygiene. An intensity of 40 microWatt /cm 2 at 253.7 nm is generally used for germicidal purposes. The eyes and skin can be damaged by exposure to direct or strongly reflected ultraviolet radiation. Adequate eye and skin protection must be worn when working in an area being irradiated. A UV-blocking face shield is the best protection for eyes and face. If a face shield is not available, safety glasses with side shields or goggles with solid side pieces may be worn for eye protection. Skin protection is afforded by face shields, caps, gloves, gowns, and other appropriate equipment.
4.3 IONIZING RADIATION
The germicidal action of x-radiation has been known for years. Gamma radiation at very high doses is used for the destruction of microorganisms in some food products. However, ionizing radiation generally is not a practical tool for laboratory use.
G. Common Research Procedures That Inactivate Biohazards
Certain experimental procedures inactivate biohazardous agents. As a consequence, subsequent work can be derated with respect to Biosafety Level. Examples of commonly used, inactivating procedures are:
- Fixation of tissues or cells as in cytogenetic studies or other fixed-cell analyses: Included here are the standard fixatives, such as 70% ethanol and mixtures of glacial acetic acid and methanol, as well as procedures that lead to extreme cross-linking of proteins, such as treatments with dimethylsuberimidate.
- Disruption of normal agent integrity, as in the extraction of DNA, RNA or protein.
- Exposure to extreme, nonphysiological conditions, e.g. pH, salt, radiation, and dessication.
NOTE: These procedures vary in their efficacy of inactivating biohazards. Derating of subsequent work should be done with caution.
H. Containment Equipment And Supplies
1. Biological Safety Cabinets
The properties and the classification of the different types of biological safety cabinets are discussed in detail in the CDC-NIH document Primary Containment for Biohazards: Selection, Installation and Use of Biological Safety Cabinets (http://www.cdc.gov/od/ohs/biosfty/bsc/bsc.htm).
The performance of biological safety cabinets will be verified at the time of installation, and thereafter on a yearly basis or whenever they are moved. This service is provided by CMO Laboratory Services. You may also contact HSEO for any question about the performance or certification of biological safety cabinets.
As with any piece of laboratory equipment, personnel must be trained in the proper use of the biological safety cabinet. Activities that disrupt the inward flow of air through the front access of the cabinet should be minimized. Repeated movement of worker's arms in and out of the work chamber, opening and closing of doors to the laboratory, improper placement or operation of materials or equipment in the work chamber, or brisk walking past the biological safety cabinet facilitate escape of aerosolized particles from within the cabinet. Use of flames should be limited. Electrical loop incinerators are recommended for bacteriological loop sterilization.
2. Containment Protection For Vacuum Systems
The aspiration of tissue culture media from monolayer cultures or of supernatants from centrifuge samples into primary collection flasks is a common laboratory procedure. Protection should be provided against drawing aerosols of hazardous chemical or biological materials or overflow fluid into the vacuum system. This protection is provided by the use of an air filter in the line immediately leading into the house vacuum line and an overflow flask for liquids between the collection flask and the air filter.
To assemble this protective apparatus, use flexible tubing of appropriate inside diameter for the flask and filter fittings and of sufficient wall thickness for the applied vacuum. Filter flasks of capacities from 250 to 4000 ml may be used for the overflow flask, depending on the available space and the amount of fluid that could be accidentally aspirated out of the collection flask. The overflow flasks should contain a disinfectant solution appropriate for the biohazardous material under study. It is essential that an antifoam agent, such as Dow Corning Antifoam A, be added to the overflow flask, since bubbling of air through the disinfectant probably will cause considerable foam which, if allowed to reach the filter, will shut off the vacuum.
If the filter becomes contaminated or requires changing, the filter and flask can be safely removed by clamping the line between filter and vacuum source. The filter and flask should be autoclaved before the filter is discarded. A new filter can be installed and the assembly replaced.
This protective apparatus for the vacuum system is shown in Fig. 1. A cartridge-type HEPA filter provides an effective barrier to passage of aerosols into the house vacuum system. The filter has a capacity to remove airborne particles 0.3 micron in size at 99.97% efficiency.
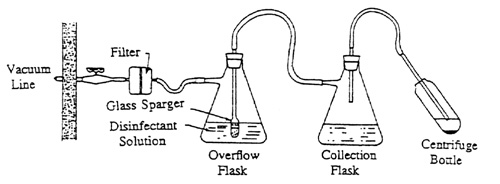
Figure 1. Protective Apparatus for the Vacuum System
I. Medical Surveillance For Biohazard Workers
Biohazard workers are defined as those staff members, contract workers, long-term visitors and students who engage in the following activities: working with any Risk Group 2 biological agents or cells (Risk Group 3 and 4 agents are NOT ALLOWED at HKUST), processing samples of tissues or body fluids (blood, serum, or semen) from humans or other primates, or handling of laboratory animals for care or experimentation. The principal investigator is responsible for informing HSEO in advance when any employee will be working with biohazardous materials. This will include work with microorganisms: isolation, culturing, mutation testing; work with virus-infected cells or isolated viruses; all forms of genetic engineering, and work involving handling of animals by animal care staff and research staff. A roster of biohazard workers will be maintained by HSEO. HSEO management must update this listing annually, and when new personnel begin work with biohazards.
Minimum medical surveillance for biohazard workers includes a review of medical records, a periodic examination of the employee’s health, with appropriate review of activities being performed and any suspected significant exposure. Medical records should include reports of any instances of accidental ingestion, inhalation, or skin penetration of biohazardous material. Employees working with potential biohazards must report any exposures immediately to HSEO. Employees being treated with immunosuppressive drugs shall be excluded from biohazard work for the duration of treatment, and the treating physician must provide a statement that the employee’s immune status is normal prior to return to biohazardous work. Modification of work regimens during pregnancy shall be determined on a case-by-case basis.
Staff who work with potentially pathogenic microorganisms, with human cells or other samples that may contain infectious agents, and those who care for or work with experimental animals, are requested to have a serum sample banked by HSEO as a baseline for future assay in the event of accidental exposure. Additional samples will be obtained for assay in the event of a suspected exposure. Blood samples will be drawn and serum prepared for storage in a labeled container in a 20oC freezer. Contact HSEO for further information.
Staff who work with human samples of any kind will be offered a course of vaccine (3 injections over 6 months) against hepatitis B virus to prevent illness in the event of accidental infection.
