Method for Testing Organic Peroxides
The presence of organic peroxide in chemicals can be tested with simple indicator paper - Whatman starch iodide paper. The Whatman starch iodide paper is sensitive to peroxide concentration below 100 ppm. Low concentration of peroxide present in chemicals turns the paper yellow whereas, high concentration of peroxide turns the paper blue. This test is sensitive to the formation of hydroperoxide which is the principal hazard associated with peroxide-forming solvent.
- Immerse the test strip in the chemical for 1 second
- Breathe slowly on the test strip for 15 to 30 seconds or until the color stabilizes (vapor in breathe provides water for the reaction to proceed)
- A yellow color indicates a low concentration of peroxide in the sample while blue color indicates a high concentration.
- If any positive results are observed, please inform HSEO immediately.
 |
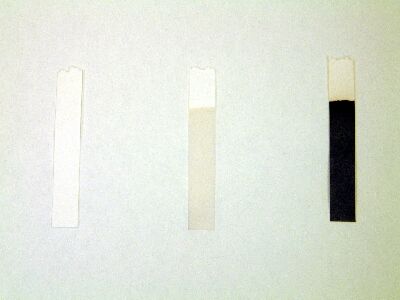 |
| Whatman Starch Iodide Test Paper |
Left: No Peroxide present - White Middle: Low concentration of peroxide - Yellow Right: High concentration of peroxide - Blue |
Reference
Review of Safety Guidelines for Peroxidizable Organic Chemicals, Chemical Health and Safety, Sept/Oct: American Chemical Society, 1996